Brain abscess: surgical experiences of 162 cases
Abstract
Aim: Brain abscess still poses a public health challenge in spite of the advent of modern neurosurgical techniques and antibiotics. Here, we present our surgical experiences and ultimate outcome in the management of brain abscess.
Methods: Totally, 162 patients with proved brain abscess who underwent surgical treatment were included in this study. The prospectively recorded data of surgical management of brain abscess and the ultimate outcome (by Glasgow outcome scale) were studied retrospectively.
Results: Total number of cases was 162, of which 113 were acute pyogenic abscess while 49 were chronic abscess. Among the chronic abscess, 29 were chronic pyogenic abscess, 14 were tubercular, 3 aspergillus, and 3 abscesses were in malignant brain metastases. In acute cases, common clinical features were headache, fever, vomiting, focal deficit and seizure. In chronic abscesses, common clinical features were mild to moderate headache and progressive focal deficit. Seventy-three (45.06%) patients had adjacent localized sinus, middle ear or cranial infection. The common predisposing factors included postneurosurgery, postpenetrating injury to brain, chronic suppurative otitis media, and congenital heart disease, infective endocarditis, sinusitis and sub optimum immuno-status. Frontal lobe involved in 30.2% cases, temporal lobe is next to involved. Single time burr hole aspiration in 111 (68.5%) cases, two or more times burr hole aspiration were done in 34 (21%) cases. Pus culture was negative in 129 (79.62%) cases. Total number of death was 22 (13.58%) cases. Complete resolution of abscess with complete recovery of preoperative neuro-deficit was seen in 80.86% cases and recovery with major neuro-deficit was observed in 5.55% cases. There is a significant association between Glasgow coma scale (GCS) on admission and mortality in brain abscess.
Conclusion: In most of the cases, pus culture did not yield growth of any causative organism. Mortality was not directly related to surgical intervention, but GCS on admission has a significant association with mortality. Early diagnosis, optimum follow-up and timely surgical interventions are the keys in the proper management of brain abscess.
Keywords
Introduction
Brain abscesses often occur in the developed world, and they are even more common in developing countries.[1] In spite of the advent of modern neurosurgical techniques, including stereotactic brain biopsy and aspiration, better culturing techniques to identify the infectious agent, new antibiotics, and modern noninvasive neuroimaging procedures, brain abscess still poses a public health challenge, especially in developing countries.[2,3] There are enormous diagnostic and therapeutic challenges and controversies in the management of brain abscess.
Here, we report our experiences including preoperative clinical features, radio-imaging findings, surgical interventions, postoperative course, complications, risk factors and causes, infectious agent and ultimate outcome in the management of brain abscess.
Methods
Totally, 162 patients with proved (peroperative and postoperative) brain abscess who underwent surgical treatment in the Department of Neurosurgery, Mitford Hospital, Dhaka Medical College Hospital, and some private hospitals (Ibn Sina specialized hospital, popular specialized hospital, Islami Bank Central Hospital and Pan Pacific Hospital) in Dhaka, Bangladesh, from July 1999 to June 2013, were included in this study. The prospectively recorded data of clinical presentation, neurological status at admission, radiological imaging, predisposing factors, anatomical location, number of lesions, surgical techniques, complications, cultured organisms, and the neurological outcome were studied by Glasgow outcome scale (GOS). Patients with evidence of neurological symptoms unrelated to brain abscess were excluded from the study as, there was evidence showing the patient had not undergone a drainage procedure or intraoperative pus sampling and the patient was lost to follow-up within the first year after operation.
Patients with features of suspected brain abscess were undergone preoperative computed tomography (CT) and/or magnetic resonance imaging (MRI) scans with contrast enhancement. The normal CT scan of brain finding was hypodense lesion with thick contrast enhancing capsule with surrounding edema. By conventional MRI, pyogenic brain abscesses were identified by hypointense signal in T1-weighted and hyperintense signal in T2-weighted, with ring-shaped enhancement and extensive surrounding edema. Conventional MRI with diffusion-weighted imaging, and magnetic resonance spectroscopy (MRS) were performed when it was difficult to discriminate brain abscesses from cystic or necrotic tumors in our later cases of the series. MRS spectra in patients with abscess showed lactate, amino acids (including valine, alanine, and leucine), and acetate peaks while spectra for patients with cystic or necrotic tumors showed only lactate peaks. Hyperintensity was detected in all the pyogenic abscess cavities, and hypointensity was observed in all the cystic and necrotic tumors on diffusion-weighted images. A predisposing factor was considered as any conditions or events which were directly related to the onset of a brain abscess. The neurological status at admission was evaluated using the Glasgow coma scale (GCS) and the outcome of the patients was assessed using the GOS on discharge and 12 months after the operation. Chi-square test was done to see the association between GCS on admission and mortality in brain abscess. Standard laboratory tests including a complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein, blood cultures, and serum chemistry were conducted in all cases. Case findings were based on the review of microbiology laboratory data for all intracranial samples. All collected intracranial pus with or without abscess wall samples were transported promptly to laboratory microscopy, aerobic, anaerobic and fungal culture and sensitivity and histopathological study. Initial empirical antimicrobial therapies were selected in accordance with the portal of entry and the anatomical location of the abscess. Initial empirical antimicrobial therapy included a combination of high dose of ceftriaxone/cefuroxime/meropenam, flucloxacillin/vancomycin and metronidazole. Between 4 and 6 days later, treatment either remained the same or was changed based on the results of antimicrobial sensitivity. Antibiotic therapy lasted for 4-8 weeks in accordance with the therapeutic response and neuroimaging findings.
Low-dose corticosteroid was used to manage perilesional edema in first 5-7 days. Seizure prophylaxis or antiepileptic medication was applied in all cases and continued for at least 2 years.
Surgical intervention
Burr hole aspiration was performed under local or general anesthesia for abscesses larger than 2.5 cm, signs of brain herniation secondary to space-occupying lesions (SOL) or ventricular proximity, abscess growth during medical therapy or SOL of uncertain etiology associated with neurological deterioration. If the size of the abscess on CT or MRI obtained after the first aspiration increased or was not reduced despite antibiotic therapy, aspiration was repeated. During surgical procedure, the abscess was drained completely and rinsed with saline containing gentamycin until the effluent was clear. Patients with poor response to repeated aspirations (with three aspirations) and medical treatment underwent complete excision of abscesses through open craniotomy excision. Postoperative abscesses where burr hole aspiration would hinder the fusion of the bone flap also underwent complete abscess excision through open craniotomy excision. Patients with otomastoiditis and brain abscess underwent radical mastoidectomy in a same time or the second session.
Results
Of 221 cases of clinico-radiologically diagnosed brain abscess, 162 cases were surgically managed. Types of abscess [Table 1], predisposing factors [Table 2], site of abscess [Table 3] and types of operations, residual neuro-deficit and outcome [Table 4] are shown.
Types of abscess
| Types of chronic abscess | Number of different type of chronic abscess | Chronic brain abscess (%) | Acute pyogenic abscess (%) | Total |
|---|---|---|---|---|
| Chronic pyogenic | 29 | 49 (30.24) | 113 (69.76) | 162 |
| Tubercular | 14 | |||
| Aspergillus | 3 | |||
| Abscess in metastases | 3 |
Predisposing factors of brain abscess
| Types of predisposing factors | Number of predisposing factors | Total predisposing factors (%) | Without predisposing factors (%) | Total |
|---|---|---|---|---|
| Postsurgery | 8 | 73 (45.06) | 89 (54.94) | 162 |
| Penetrating injury | 11 | |||
| CSOM/mastoiditis | 22 | |||
| Congenital heart disease | 10 | |||
| Infective endocarditis | 3 | |||
| Frontal sinusitis | 12 | |||
| Ethmoidal sinusitis | 4 | |||
| Immunocompromised | 3 |
Site distribution
| Types of abscess | Frontal | Temporal | Parietal | Occipital | Cerebellar | Ganglio-thalamic zone |
|---|---|---|---|---|---|---|
| Acute pyogenic (113) | 34 | 25 | 16 | 18 | 13 | 7 |
| Chronic pyogenic (29) | 10 | 9 | 4 | 3 | 3 | 0 |
| Tubercular (14) | 3 | 3 | 2 | 1 | 4 | 1 |
| Aspergillus (3) | 2 | - | - | 1 | - | - |
| Abscess in metastesis (3) | - | - | - | 1 | 1 | 1 |
| Total (%) | 49 (30.2) | 37 (22.8) | 22 (13.6) | 24 (14.8) | 21 (12.96) | 9 (5.5) |
Types of operations, residual neuro-deficit and outcome
| Operations | Number | Mortality | Residual major neuro-deficit | Complete recovery |
|---|---|---|---|---|
| Single burr hole aspiration | 111 (68.5%) | 11 | 4 (hemiparesis, motor dysphasia, hand weakness, footdrop) | 96 |
| Multiple aspiration | 34 (21%) | 9 | 3 (monoparesis, sensory dysphasia, visual field defect) | 22 |
| Third ventriculoscopic (endoscopic) drainage and ETV [Figure 6] | 1 (0.62%) | 0 | 0 | 1 |
| Excision of abscess by craniotomy | 16 (9.87%) | 2 | 2 (nominal dysphasia, monoparesis) | 12 |
| Total | 162 | 22 (13.58%) | 9 (5.55%) | 131 (80.86%) |
One hundred and thirteen cases were acute pyogenic abscess [Figures 1-4] and 49 were chronic abscess. Among the chronic abscess, 29 were chronic pyogenic abscess, 14 were tubercular [Figures 5 and 6], 3 aspergillus [Figure 7] and 3 abscesses were in malignant brain metastases.
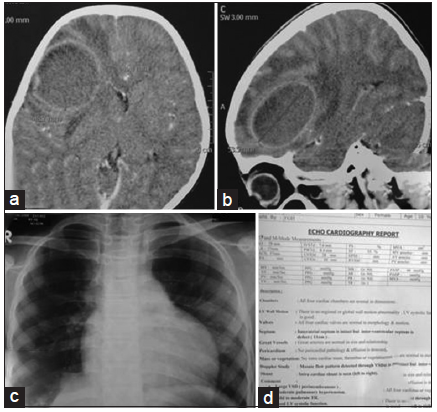
Figure 1. Preoperative contrast computed tomography scan of brain (a: axial section; b: sagittal section) showing right-sided posterior frontal brain abscess in child with tetralogy of Fallot; (c) X-ray chest P/A view showing "boot shaped" heart shadow; (d) echocardiogram report
Figure 2. (a) Preoperative contrast CT axial section and (b) preoperative contrast MRI showing left sided paraventricular abscess with mass effect and edema; (c) posttreatment MRI of brain in T1W axial section and (d) posttreatment MRI axial section in fluid-attenuated inversion recovery showing complete resolution of abscess with some gliosis and cerebromalacia. CT: computed tomography; MRI: magnetic resonance imaging
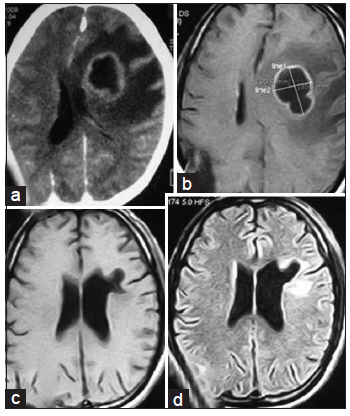
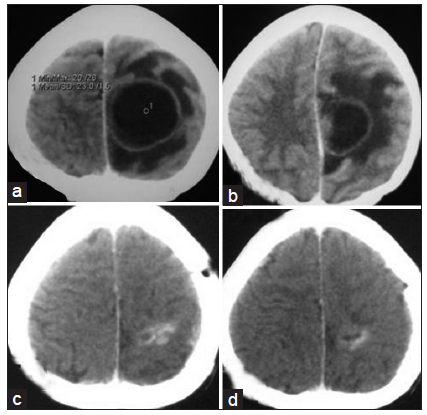
Figure 3. (a and b) Contrast CT scan of brain axial section showing left sided parasagital fronto-parietal abscess; (c and d) contrast CT scan of brain after completion of treatment resolution of abscess with some residual calcification. CT: computed tomography
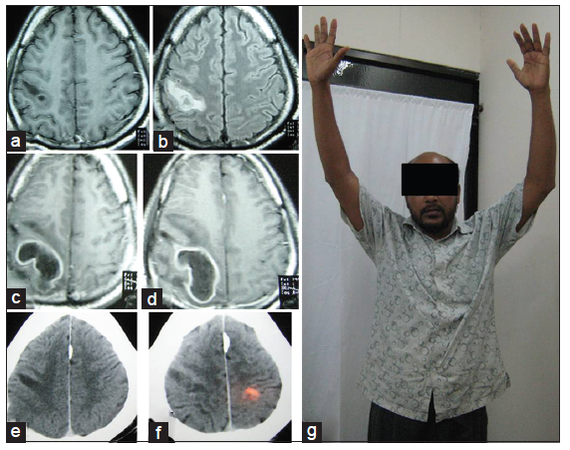
Figure 4. (a and b) MRI of brain axial sections, contrast and fluid-attenuated inversion recovery images respectively was done after sudden development of left sided hemiplegia showing noncontrast enhancing hypointense lesion in precentral gyrus; (c and d) contrast MRI of brain axial sections, 16 days after initial presentation showing ring enhancing lesion (abscess); (e and f) (contrast CT scan axial section 3 months after operation) showing resolution of abscess with some gliosis; (g) patient-3 months after operation (with full neurological recovery). CT: computed tomography; MRI: magnetic resonance imaging
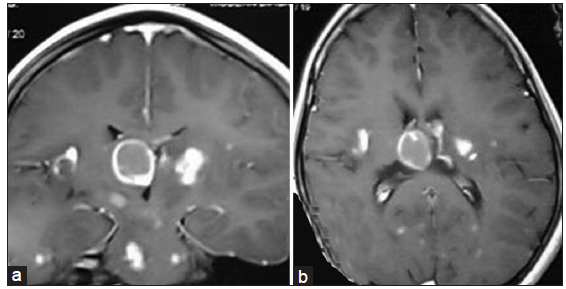
Figure 5. (a) Coronal section and (b) axial section showing tubercular abscess a b in right lateral ventricular subcallosal region with multiple satellite tubercular lesion throughout the brain
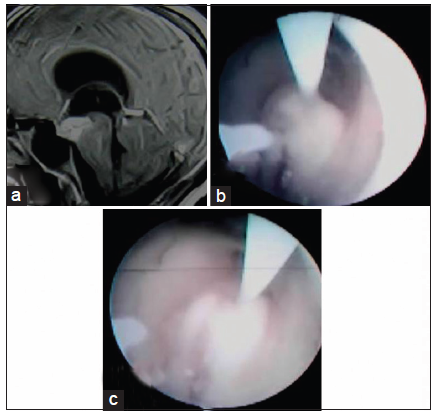
Figure 6. (a) Preoperative contrast magnetic resonance imaging of brain in sagittal section showing contrast enhancing lesion (tubercular abscess) in third ventricular floor and interpeduncular fossa; (b and c) per operative picture during endoscopic third ventriculoscopic interventions showing emergence of tubercular pus from the lesion
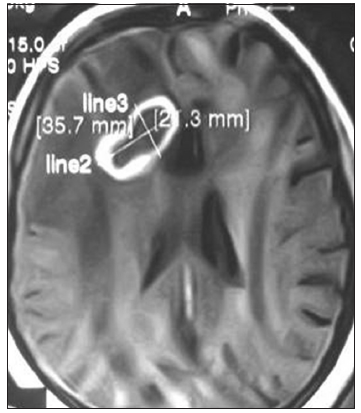
Figure 7. Contrast magnetic resonance imaging of brain axial section showing ring enhancing right frontal aspergillus abscess (proved by postoperative culture of pus and histopathology) with perilesional edema
Age range was 3-72 (average 42.5) years. The male-to-female ratio in our study was 3.37:1. Gender distribution, numbers of abscess and laboratory findings of patients are shown in Table 5.
Gender distribution, number of abscess and laboratory findings of patients
| Demographic variables | Number (%) |
|---|---|
| Gender | |
| Male | 125 (77.16) |
| Female | 37 (22.84) |
| Raised lab parameters | |
| ESR | 41 (25.35) |
| CRP | 84 (51.85) |
| WBC | 78 (48.14) |
| Number of abscess | |
| Single | 126 (77.7) |
| Multiple | 36 (22.3) |
In acute cases common clinical features were headache (89.3%), fever (67.5%), vomiting (38%), focal deficit (31%) and seizure (22.6%) focal and secondary generalized). Among the chronic pyogenic cases, there was a history of acute febrile illness in 15 cases (out of 29; 51.7%). In all chronic abscesses, common clinical features were mild to moderate headache and progressive focal deficit. In tubercular abscess, clinical features were low-grade fever, weight loss and anorexia in addition to headache. Two patients with tubercular abscess in temporal lobe presented with temporal lobe epilepsy and superior orbital fissure syndrome. Concurrent tuberculosis in another system was found only in 3 out of 14 cases of tubercular abscess. In aspergillus abscess, 1 patient was with renal transplant and 2 were SLE patients [Figure 7]. No primary site for malignancy was found in those 3 brain abscesses in metastasis.
There was hemiparesis in 52 cases, hemiplegia in 23 cases, monoplegia in 12 cases, monoparesis in 19 cases, motor aphasia in 14 cases, dysphasia in 13 cases, and sensory aphasia in 17 cases. Visual disturbances were found in 11 cases (especially in occipital lobe abscess). There was short-term memory loss in 5 cases, bowel and bladder incontinence in 3 cases, frontal lobe syndrome in 4 cases, temporal lobe epilepsy in 21 cases, and gait disturbances in 19 cases. There was coarse hemi tremor in 1 case. In 2 patients, presentations were like that of acute stroke [Figure 4].
Seventy-three (45.06%) patients had adjacent localized cranial infection, chronic suppurative otitis media (CSOM) or paranasal sinusitis. The most common predisposing factors included postneurosurgery (8 cases), postpenetrating injury to brain (11 cases), CSOM (22 cases), and congenital heart disease (in 10 patients including 4 cases of Tetralogy of Fallot-TOF), infective endocarditis (3 cases), frontal sinusitis (12 cases), ethmoidal sinusitis (4 cases), and 3 patients were immunosuppressed or immunocompromised.
Frontal lobe involved in 49 (30.2%) cases of brain abscess, temporal lobe is next to involved in 37 (22.8%) cases. Parietal, occipital, cerebellar and gangliothalamic zone in 22 (13.6%), 24 (14.8%), 21 (21.96%) and 9 (5.5%) cases respectively. Site distributions of brain abscess were shown in Table 3.
Operations used in brain abscess surgery were single time burr hole aspiration in 111 (68.5%) cases, two or more times burr hole aspiration in 34 (21%) cases, excision of abscess by craniotomy in 16 (9.87%) cases and third ventriculoscopic (endoscopic) tubercular abscess drained with endoscopic third ventriculostomy) in third ventricular floor tubercular abscess in one (0.62%) cases [Figure 6]. Types of operations, residual neuro-deficit, mortality and outcome are illustrated in Table 4. Pus culture indicated negative results in 145 (89.5%) cases. Anaerobic culture and culture for Mycobacterium failed to yield any bacterial growth. Organisms isolated from pus culture are shown in Table 6.
Culture-positive bacterial-fungal isolates from brain abscesses
| Bacteria-fungus | Number of patients | Percentage |
|---|---|---|
| Streptococcus intermedius | 2 | 10.5% |
| Ps. Aeruginosa | 3 | |
| Staphylococcus areus | 4 | |
| Streptococus epidermidis | 1 | |
| Streptococcus pyogenes | 3 | |
| Streptococcus pneumoniae | 1 | |
| Mycobacterium | 0 | |
| Anarobic | 0 | |
| Fungal | 3 | |
| No growth | 145 | 89.5% |
Total number of death was 22 (13.58%) cases. Complete resolution of an abscess with complete recovery of preoperative neuro-deficit was observed in 131 (80.86%) cases [Figures 2-4]. Complete resolution of an abscess with residual preoperative major neuro-deficit was detected in 9 (5.55%) cases. Persistent major neuro-deficit was hemiparesis 1, motor dysphasia 1, hand weakness 1, foot drop 1, monoparesis 2, sensory dysphasia 1, nominal dysphasia and visual field defect 1. Coarse hemi-tremor resolved postoperatively along with abscess resolution. Mortality and morbidity with GCS at admission and GOS on last follow-up are shown in Table 7. Patients GCS on admission had a significant effect on mortality in brain abscess as shown in Table 8. Six patients with congenital heart diseases underwent cardiac surgery; sinus surgery was performed in 12 patients and 5 patients underwent mastoidectomy in a different sitting within 1 year after brain abscess surgery without any mortality.
Mortality and morbidity with GCS at admission and GOS on last follow‑up
| GCS on admission | Number | Mortality | Major residualneurodeficit | GOS on last follow-up | Number (%) |
|---|---|---|---|---|---|
| 3-7 | 11 (6.8%) | 7 | 1 | 1 (death) | 13 (8.02) |
| 8-12 | 29 (17.9%) | 5 | 1 | 2 (vegetative) | 0 (0) |
| 13-15 | 122 (75.3%) | 10 | 7 | 3 (severe disability) | 0 (0) |
| 4 (moderate disability) | 9 (5.55) | ||||
| Total | 162 | 22 (13.58%) | 9 (5.55%) | 5 (good recovery) | 131 (80.86) |
Relationship between mortality and GCS score on admission in brain abscess
| GCS on admission | Total number of patient | Total mortality | Total survivality | Chi-square test |
|---|---|---|---|---|
| 3-12 | 40 | 12 | 28 | 12.2 (at 5% level of significance calculated Chi-square test value 12.2 is greater than table value 3.84) |
| 13-15 | 122 | 10 | 112 | |
| Total | 162 | 22 | 140 |
Discussion
Brain abscess is an intraparenchymal collection of pus. The incidence of brain abscesses is about 8% of intracranial masses in developing countries, whereas, in Western countries, the incidence is about 1-2%.[1,4-6] Though potentially curable, there was still a diagnostic and therapeutic challenge. In the last two decades, there is a major advance in the diagnosis and management of brain abscesses, with a corresponding improvement in the survival rate. In the development of brain abscess, inoculation of an organism is required into the brain parenchyma in an area of devitalized brain tissue or in a region with poor microcirculation, and the lesion evolves from an early cerebritis stage to the stage of organization and capsule formation.[7] Histologically, there are four stages in brain abscess formation: early cerebritis (day 1-3), late cerebritis (day 4-9), early encapsulation (day 10-13) and late capsule stage (day 14 onward). About 2 weeks are required for encapsulation, which is usually less complete on medial or ventricular side due to poor vascular supply.[8,9] The mode of entry of organisms could be by contiguous spread, hematogenous dissemination, or following trauma.[4] The common predisposing factors of a brain abscess are CSOM, congenital cyanotic heart disease, and paranasal sinusitis.[1,5,10-12]
Immunosuppression due to disease or therapy is emerging as an important risk factor for the development of brain abscess.[4] Here, we found predisposing factors of brain abscesses were similar.
The most common organism isolated from a brain abscess was Staphylococcus aureus in the preantibiotic era.[5] Now, Streptococcus spp. have replaced Staphylococcus spp. as the most common organisms.[5,13] Based on the site of origin, the organisms would be different. Streptococci were isolated from abscesses of all types and at all sites, whereas Enterobacteriaceae and Bacteroides spp. were isolated from otogenic temporal lobe abscesses, which had mixed cultures.[13]Streptococcus spp. have been most commonly isolated from cardiogenic abscesses.[14] In neonates, the most common organisms are Proteus and Citrobacter spp. Anaerobes are one of the most common causative organisms in a brain abscess.[15] Polymicrobial infections are common, indicating the importance of using both aerobic and anaerobic cultures in diagnosis.[15,16] Cultures for acid-fast bacilli and fungi should be conducted in all cases as occasionally, intracranial tuberculosis as well as fungal infections can present as an abscess.[17-20] In our series, majority of the culture failed to show positive bacterial growth. More than one-third of otogenic and metastatic abscesses are polymicrobial (aerobic and/or anaerobic). Bacteroides, peptostreptococcus and fusobacterium are common anaerobes and are sensitive to metronidazole.[21-23] Rhinogenic abscess is generally streptococcal. Staphylococcus is common in posttraumatic and postoperative cases. In infants and neonates, postmeningitic abscess is caused by Gram-negative organisms.[24]
Clinically, brain abscess presents with features of rapidly expanding intracranial mass lesion that is, raised intracranial pressure (ICP) in the form of constant progressive headache refractory to therapy, vomiting, papilledema, focal deficits, convulsions, meningism and altered sensorium. The classical triad of headache, focal neurological deficits and fever is found in 25% cases only.[5] Brain abscess occurs in the younger age groups usually in the first three decades of life.[1,6] Seizures have been reported in up to 50% of cases.[1,6,25] The duration of symptoms is usually < 2 weeks, with rapid onset and progression. Immunocompromised patients may have an insidious onset.[6] Three patients in this series had bladder and bowel incontinence; one had tubercular abscess in third ventricular floor with hydrocephalus, second one had one large abscess in frontal lobe and another abscess in cerebritis stage on the opposite frontal lobe and the rest had large paracentral lobule abscess with huge mass effect compressing the opposite side.
A lumbar puncture is contraindicated in patients with a suspected brain abscess because it can result in transtentorial or transforaminal herniation and subsequent death.[26] CT facilitates early detection, exact localization, accurate characterization, determination of number, size and staging of the abscess. It also detects hydrocephalus, raised ICP, edema and associated infections like subdural empyema and thus helps in treatment planning. It is invaluable in the assessment of the adequacy of treatment and sequential follow-up. An ill-defined area of low density, on plain CT, corresponds to developing necrotic center in the cerebritis stage.[6] In the early capsule stage, a slightly hyperdense, faint ring is seen surrounding a necrotic hypodense center. With contrast, the ring shows thin regular enhancement of uniform thickness and smooth contour on its inner surface with marked perilesional hypodense area suggestive of edema. In the late capsule stage, the capsule is seen as a ring in plain CT. With contrast, it shows thick enhancement gradually fading in delayed scans. Ring enhancement can be seen in the late cerebritis stage and is not an absolute evidence of encapsulation.[8,9] Radiological features alone are inadequate to differentiate pyogenic brain abscess from fungal, nocardial or tuberculous abscess, inflammatory granuloma (tuberculoma), neurocysticercosis, toxoplasmosis, metastasis, glioma, resolving haematoma, infarct, hydatid cyst lymphoma and radionecrosis.[27-30] However, fever, meningism, raised ESR, multilocularity, leptomeningeal or ependymal enhancement, reduction of ring enhancement in delayed scan and finding of gas within the lesion favor a diagnosis of abscess.[9] Positive labeling in radionuclide imaging with III-Indium labeled leukocytes, C-reactive protein, 99m TC-hexamethylpropylene amine oxime leukocyte scintigraphy, diffusion weighted MRI, Thallium-201 single photon emission CT, and proton MRS are helpful in differentiating abscess from tumor.[31-38]
Brain abscesses were singular in 77.7% of the subjects and multiple in 22.3%, a result similar to that reported by Landriel et al.[39] The frontal lobe was the most common abscess location in the patients, followed by the temporal and occipital regions. However, in a study carried out by Cavusoglu et al.,[40] the temporoparietal region was the most commonly affected location. Abscesses of unknown cause accounted for 54.94% of the subjects, higher than the values reported for other series.[12,39,41-43] In most large series of brain abscesses from developing countries, middle ear infection has been reported to be the most common source of intracranial suppuration, a result similar to the current study.[44]
The basic principle of treatment is the prescription of appropriate antibiotics with or without aspiration, treatment of sequelae that is, hydrocephalus, seizures etc., and eradication of primary source.[26] According to a number of authors, treatment of brain abscess involves aspiration of the pus or excision of the abscess, followed by parenteral antibiotic therapy.[1,5,6,12,45] Empirical medical therapy is the best avoided and should be reserved for patients in whom a bacteriological diagnosis has been obtained from a systemic source or who are extremely ill that is, too ill to undergo any forms of intervention.[45] Small abscesses and lesions in the cerebritis stage respond well to medical therapy alone.[14] The choice between conservative versus operative treatment is influenced by age, neurological status, location, number, size and stage of abscess formation. Each case must be individualized and treated on its own merits. Conservative treatment can be tried in patients who are alert, clinically stable and have a major risk for surgery and anesthesia. Treatment of sequelae that is, hydrocephalus, seizures, etc., and eradication of primary source should not be neglected. The management should be done by neurosurgeons prepared to operate at the first sign of failure of medical therapy or where immediate neurosurgical help is available. Medical treatment alone should not be applied when the diagnosis is not yet confirmed. Abscess in cerebritis stage, or walled off but smaller than 3 cm diameter could be treated nonsurgically with antibiotics alone.[27] Serial CT scans are crucial as it may enlarge despite adequate antibiotics therapy.[46] The complexity of microbial flora in brain abscess necessitates empirical antibiotic therapy against both aerobic and anaerobic organisms. Usually, intravenously administration of “triple high dose” antibiotics for 2 weeks followed by 4 weeks of oral therapy is recommended. Corticosteroid can only be used to reduce edema and administration of anticonvulsant should be routine in supratentorial abscess, but duration is a matter of debate.[6]
If serial CT scans show increased size of abscess at any time during conservative treatment with antibiotics or no decrease in size within 4 weeks, a surgical procedure should be performed to confirm the diagnosis, to obtain a sample for culture of identification of specific pathogens and sensitivity to particular antibiotics, and to remove as much purulent material as possible. Walled off abscess larger than 3 cm diameter and a smaller deep-seated white matter abscess are unlikely to respond medical treatment alone. Standard therapy for such lesions should be surgical evacuation followed by appropriate antibiotic.[47] Instillation of antibiotics inside the abscess cavity can be considered. A surgical drainage allows immediate decompression of mass lesion and reduction of ICP that reduces the duration of antibiotic therapy and hospitalization. It increases the likelihood of cure. Surgery should be performed in case of clinical deterioration, significant mass effect and neurological deficit. Many surgical techniques have been developed, but there is no single best method.[48] At present, aspiration and excision are two common procedures used. Role of aspiration versus excision is controversial. In choosing between aspiration and excision, various factors including surgical morbidity, success rate and sequelae such as recurrence and seizure disorders also must be considered. Aspiration is a rapid and safe procedure, especially with the use of stereotactic techniques, ultrasound or CT scan guidance. It can be done under local anesthesia, on bedside, even in seriously ill or high-risk patients. Aspiration can be done at any stage of evolution of abscess. If no pus is obtained, biopsy gives positive culture even in early cerebritis stage. A large, superficial, or accessible abscess can be aspirated via appropriately placed burr hole. Real time ultrasound, particularly in infants with open fontanelle and stereotaxy provides precise localization. Free hand needle aspiration can be a very effective life-saving measure in the underdeveloped world where stereotaxy is not available.[30] More than one aspiration may be required. Repeat aspirations are often necessary for cure.
With free availability of CT scan, role of aspiration has increased, as abscesses can be detected easily and follow up is available immediately. Some authors recommended stereotactic aspiration/biopsy in all patients with suspected brain abscess regardless of size.[49] Aspiration has a place, both as preparatory to eventual excision (secondary excision) and as a definitive procedure.[48] Multiloculated abscesses have been treated with stereotactic aspiration of all loculi in single or staged aspiration. Encouraging results with endoscopic stereotactic evacuation of brain abscess has been shown recently.[50] Neuroendoscopic treatment, when compared to stereotactic aspiration, has an additional advantage of more complete drainage and lavage.[51]
Many authors recommended craniotomy and excision for abscesses that enlarge after 2 weeks of antibiotic therapy or that fail to shrink after 3-4 weeks of antibiotics.[1,6,7,45] Craniotomy is also recommended for multiloculated abscesses and larger lesions with significant mass effect that are superficial and located in noneloquent regions of the brain. A few authors also recommended excision of abscesses in the cerebellum, where recurrent pus collection following aspiration can lead to precipitous neurological worsening.[26] There are certain advantages to excision of a brain abscess in an otherwise neurologically intact patient. The risk of repeated collection of pus is almost completely eliminated, and hence the expense involved in repeated imaging is saved. The duration of hospitalization is also reduced. Furthermore, in patients with an otogenic brain abscess, the disease in the middle ear can also be surgically treated at the same sitting or soon thereafter.[18] This also reduces the likelihood of recurrence of the abscess. Abscess resulting from fistulous communication, example, trauma and congenital dermal sinus, require excision of infected granulation tissue and closure of the fistula. Abscess localized to one lobe and contiguous to primary source that is, frontal sinus osteomyelitis, is better treated with excision along with the primary focus. Posttraumatic abscess containing foreign body or contaminated retained bone fragments requires excision to prevent recurrence.[8,46] Abscesses containing gas are resistant to antibiotics and are better treated with excision.[52] Large superficial abscesses resistant to multiple aspirations and not showing volume reduction because of adhesions to the dura, due to large brain surface area should be excised for cure. Multiloculated actinomycotic and nocardial abscess may need excision as simple aspiration may prove inadequate.[53] Excision reduces the incidence of seizures and prevents recurrence. Abscess in cerebritis stage, deep-seated abscesses in eloquent areas and multiple abscesses are the situation where excision should not be considered.[6]
The mortality rate in our study was 13.58% (22 cases). Sixteen patients died in the immediate postoperative period from brain herniation with very high ICP (15 cases), ARDS (2 cases), septicemia with systemic inflammatory response, multiple organ dysfunction (4 cases) and one patient died from acute pancreatitis after operation. The mortality rate shown here is similar to the rates observed by other authors, which range between 8% and 53%.[54] Manzar et al.[43] reported that the most important factors influencing mortality was the neurologic condition of the patient at the time of admission. Here, we found mortality was very high in brain abscess with low GCS score on admission [Tables 7 and 8]. Landriel et al.[39] revealed that age, immunosuppression and hematogenous spread were all associated with poor outcomes.
In conclusion, predisposing factors were seen in nearly half of the cases. In most of the cases, pus culture did not yield causative organisms. From this series, we see that in “chronic abscess group” pyogenic abscess were the commonest followed by tuberculus abscess but possibilities of other causes (i.e. fungal) should not be overlooked. Mortality due to brain abscess was not directly related to surgical intervention but on admission GCS has a significant association with the mortality. Like other diseases, we can state early diagnosis and optimum follow-up, and timely surgical interventions are the keys in the management of brain abscess.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1. Bernardini GL. Diagnosis and management of brain abscess and subdural empyema. Curr Neurol Neurosci Rep 2004;4:448-56.
2. Wilson HL, Kennedy KJ. Scedosporium apiospermum brain abscesses in an immunocompetent man with silicosis. Med Mycol Case Rep 2013;2:75-8.
3. Ansari MK, Jha S. Tuberculous brain abscess in an immunocompetent adolescent. J Nat Sci Biol Med 2014;5:170-2.
4. Zhang C, Hu L, Wu X, Hu G, Ding X, Lu Y. A retrospective study on the aetiology, management, and outcome of brain abscess in an 11-year, single-centre study from China. BMC Infect Dis 2014;14:311.
5. Loftus CM, Osenbach RK, Biller J. Diagnosis and management of brain abscess. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996. pp. 3285-98.
6. Sharma BS, Gupta SK, Khosla VK. Current concepts in the management of pyogenic brain abscess. Neurol India 2000;48:105-11.
7. Takeshita M, Kagawa M, Yonetani H, Izawa M, Yato S, Nakanishi T, Monma K. Risk factors for brain abscess in patients with congenital cyanotic heart disease. Neurol Med Chir (Tokyo) 1992;32:667-70.
8. Joshi SM, Devkota UP. The management of brain abscess in a developing country: are the results any different? Br J Neurosurg 1998;12:325-8.
9. Britt RH. Brain abscess. In: Wilkins RH, Rengachary SS, editors. Neurosurgery New York: McGraw-Hill; 1985. pp. 1928-56.
10. Loeffler JM, Bodmer T, Zimmerli W, Leib SL. Nocardial brain abscess: observation of treatment strategies and outcome in Switzerland from 1992 to 1999. Infection 2001;29:337-41.
11. Malik S, Joshi SM, Kandoth PW, Vengsarkar US. Experience with brain abscesses. Indian Pediatr 1994;31:661-6.
12. Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol 2006;65:557-62.
13. de Louvois J, Gortavai P, Hurley R. Bacteriology of abscesses of the central nervous system: a multicentre prospective study. Br Med J 1977;2:981-4.
14. Townsend GC, Scheld WM. Infections of the central nervous system. Adv Intern Med 1998;43:403-47.
15. Engelhardt K, Kampfl A, Spiegel M, Pfausler B, Hausdorfer H, Schmutzhard E. Brain abscess due to Capnocytophaga species, Actinomyces species, and Streptococcus intermedius in a patient with cyanotic congenital heart disease. Eur J Clin Microbiol Infect Dis 2002;21:236-7.
16. de Louvois J. Bacteriological examination of pus from abscesses of the central nervous system. J Clin Pathol 1980;33:66-71.
17. Dash K, Dash A, Pujari S, Das B, Devi K, Mohanty R. Bilateral mycotic cerebral abscess due to aspergillosis – a case report. Indian J Pathol Microbiol 2006;49:555-7.
18. Kumar R, Pandey CK, Bose N, Sahay S. Tuberculous brain abscess: clinical presentation, pathophysiology and treatment (in children). Childs Nerv Syst 2002;18:118-23.
19. Mohanty A, Venkatarama SK, Vasudev MK, Khanna N, Anandh B. Role of stereotactic aspiration in the management of tuberculous brain abscess. Surg Neurol 1999;51:443-6.
20. Mohindra S, Mohindra S, Gupta R, Bakshi J, Gupta SK. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses 2007;50:290-6.
21. Rosenblum ML, Mampalam TJ, Pons VG. Controversies in the management of brain abscesses. Clin Neurosurg 1986;33:603-32.
22. Grigoriadis E, Gold WL. Pyogenic brain abscess caused by Streptococcus pneumoniae: case report and review. Clin Infect Dis 1997;25:1108-12.
23. Stroobandt G, Zech F, Thauvoy C, Mathurin P, de Nijs C, Gilliard C. Treatment by aspiration of brain abscesses. Acta Neurochir (Wien) 1987;85:138-47.
24. Chaudhry R, Dhawan B, Laxmi BV, Mehta VS. The microbial spectrum of brain abscess with special reference to anaerobic bacteria. Br J Neurosurg 1998;12:127-30.
25. Bagdatoglu H, Ildan F, Cetinalp E, Doganay M, Boyar B, Uzuneyüpoglu Z, Haciyakupoğlu S, Karadayi A. The clinical presentation of intracranial abscesses. A study of seventy-eight cases. J Neurosurg Sci 1992;36:139-43.
26. Unnikrishnan M, Chandy MJ, Abraham J. Posterior fossa abscesses. A review of 33 cases. J Assoc Physicians India 1989;37:376-8.
27. Bhatia R, Tandon PN, Banerji AK. Brain abscess – an analysis of 55 cases. Int Surg 1973;58:565-8.
28. Miller ES, Dias PS, Uttley D. CT scanning in the management of intracranial abscess: a review of 100 cases. Br J Neurosurg 1988;2:439-46.
29. Rajshekhar V, Chandy MJ. Computerized tomography-guided stereotactic surgery for brainstem masses: a risk-benefit analysis in 71 patients. J Neurosurg 1995;82:976-81.
31. Young RF, Frazee J. Gas within intracranial abscess cavities: an indication for surgical excision. Ann Neurol 1984;16:35-9.
32. Ogg G, Lynn WA, Peters M, Curati W, McLaughlin JE, Shaunak S. Cerebral nocardia abscesses in a patient with AIDS: correlation of magnetic resonance and white cell scanning images with neuropathological findings. J Infect 1997;35:311-3.
33. Vogelsang JP, Wehe A, Markakis E. Postoperative intracranial abscess – clinical aspects in the differential diagnosis to early recur rence of malignant glioma. Clin Neurol Neurosur 1998;100:11-4.
34. Grimstad IA, Hirschberg H, Rootwelt K. 99mTc - hexamethylpropyleneamine oxime leukocyte scintigraphy and C-reactive protein levels in the differential diagnosis of brain abscesses. J Neurosurg 1992;77:732-6.
35. Saba PR, McDonald K, Hunn J, Dastur K, Chablani V. Technetium-99m HMPAO labeled leukocytes as the primary diagnostic tool in a case of brain abscess. Clin Nucl Med 1997;22:679-82.
36. Kim YJ, Chang KH, Song IC, Kim HD, Seong SO, Kim YH, Han MH. Brain abscess and necrotic or cystic brain tumor: discrimination with signal intensity on diffusion-weighted MR imaging. AJR Am J Roentgenol 1998;171:1487-90.
37. Dev R, Gupta RK, Poptani H, Roy R, Sharma S, Husain M. Role of in vivo proton magnetic resonance spectroscopy in the diagnosis and management of brain abscesses. Neurosurgery 1998;42:37-42.
38. Rémy C, Grand S, Laï ES, Belle V, Hoffmann D, Berger F, Estève F, Ziegler A, Le Bas JF, Benabid AL. 1H MRS of human brain abscesses in vivo and in vitro. Magn Reson Med 1995;34:508-14.
39. Landriel F, Ajler P, Hem S, Bendersky D, Goldschmidt E, Garategui L, Vecchi E, Konsol O, Carrizo A. Supratentorial and infratentorial brain abscesses: surgical treatment, complications and outcomes – a 10-year single-center study. Acta Neurochir (Wien) 2012;154:903-11.
40. Cavusoglu H, Kaya RA, Türkmenoglu ON, Colak I, Aydin Y. Brain abscess: analysis of results in a series of 51 patients with a combined surgical and medical approach during an 11-year period. Neurosurg Focus 2008;24:E9.
41. Xiao F, Tseng MY, Teng LJ, Tseng HM, Tsai JC. Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol 2005;63:442-9.
42. Kothari M, Goel A. Brain abscess: a cogent clarifier of the confused concept of immunity. Neurosurg Focus 2008;24:E16.
43. Manzar N, Manzar B, Kumar R, Bari ME. The study of etiologic and demographic characteristics of intracranial brain abscess: a consecutive case series study from Pakistan. World Neurosurg 2011;76:195-200.
44. Osma U, Cureoglu S, Hosoglu S. The complications of chronic otitis media: report of 93 cases. J Laryngol Otol 2000;114:97-100.
45. Arunkumar MJ, Rajshekhar V, Chandy MJ, Thomas PP, Jacob CK. Management and outcome of brain abscess in renal transplant recipients. Postgrad Med J 2000;76:207-11.
46. Chang KH, Song IC, Kim SH, Han MH, Kim HD, Seong SO, Jung HW, Han MC. In vivo single-voxel proton MR spectroscopy in intracranial cystic masses. AJNR Am J Neuroradiol 1998;19:401-5.
47. Chacko AG, Chandy MJ. Diagnostic and staged stereotactic aspiration of multiple bihemispheric pyogenic brain abscesses. Surg Neurol 1997;48:278-82.
49. Kondziolka D, Duma CM, Lunsford LD. Factors that enhance the likelihood of successful stereotactic treatment of brain abscesses. Acta Neurochir (Wien) 1994;127:85-90.
50. Hellwig D, Benes L, Bertalanffy H, Bauer BL. Endoscopic stereotaxy – an eight year's experience. Stereotact Funct Neurosurg 1997;68 (1-4 Pt 1):90-7.
51. Fritsch M, Manwaring KH. Endoscopic treatment of brain abscess in children. Minim Invasive Neurosurg 1997;40:103-6.
52. Cohen JE, Mierez R, Tsai EC. Postcraniotomy gas-containing brain abscess: a neurosurgical emergency. Case report. Surg Neurol 1999;51:568-70.
53. Oktem IS, Akdemir H, Sümerkan B, Koç RK, Menkü A, Tümtürk F. Cerebellar abscess due to Nocardia asteroides. Acta Neurochir (Wien) 1999;141:217-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Chowdhury FH, Haque MR, Sarkar MH, Chowdhury SMNK, Hossain Z, Ranjan S. Brain abscess: surgical experiences of 162 cases. Neurosciences 2015;2:153-61. http://dx.doi.org/10.4103/2347-8659.160851
AMA Style
Chowdhury FH, Haque MR, Sarkar MH, Chowdhury SMNK, Hossain Z, Ranjan S. Brain abscess: surgical experiences of 162 cases. Neuroimmunology and Neuroinflammation. 2015; 2: 153-61. http://dx.doi.org/10.4103/2347-8659.160851
Chicago/Turabian Style
Chowdhury, Forhad Hossain, Md Raziul Haque, Mainul Haque Sarkar, S. M. Noman Khaled Chowdhury, Zahed Hossain, Shisir Ranjan. 2015. "Brain abscess: surgical experiences of 162 cases" Neuroimmunology and Neuroinflammation. 2: 153-61. http://dx.doi.org/10.4103/2347-8659.160851
ACS Style
Chowdhury, FH.; Haque MR.; Sarkar MH.; Chowdhury SMNK.; Hossain Z.; Ranjan S. Brain abscess: surgical experiences of 162 cases. Neurosciences. 2015, 2, 153-61. http://dx.doi.org/10.4103/2347-8659.160851
About This Article
Copyright
Data & Comments
Data
 Cite This Article 18 clicks
Cite This Article 18 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.