Intrathecal dexamethasone and methotrexate treatment of neoplastic meningitis from solid tumors
Abstract
Aim: Neoplastic meningitis (NM) from solid tumors is an advanced malignancy with poor prognosis. Intrathecal chemotherapy is a reliable treatment, and we have obtained some experiences in the treatment of NM with intrathecal dexamethasone and methotrexate (IT DXM and MTX).
Methods: Retrospective study of 23 patients with NM from lung cancer (n = 11), breast cancer (n = 3), gastric cancer (n = 1), malignant melanoma (n = 1), unknown cancer (n = 7) was conducted. Among these patients, eight received IT DXM and MTX treatment, and 15 patients were placed into a palliative care group. Overall survival (OS) was compared, and the patients’ characteristics, symptoms, and some laboratory examinations were analyzed to find the risk factors affecting OS.
Results: OS of IT DXM and MTX group was significantly longer than that of the palliative care group (P = 0.01). The median survival (MS) of palliative care group was 7.53 weeks (5.50-9.57; n = 15), and of the IT DXM and MTX group, 28.63 weeks (12.50-44.75; n = 8); IT DXM and MTX prolonged the OS of NM patients (regression coefficient = −2.923), with odds ratio (OR) being 0.054 (0.09-0.323). Spinal nerves damage decreased the OS (regression coefficient = 1.595), with OR being 4.928 (1.382-17.579).
Conclusion: IT DXM and MTX have prolonged the patients’ MS, which could be used as a fundamental treatment of NM. Time of induction treatment should be flexible and individualized, and induction treatment could restart when central nervous system relapse. Patients with spinal nerves damage are apt to live shorter.
Keywords
Introduction
Neoplastic meningitis (NM) is the leptomeningeal dissemination of metastatic tumors; a devastating complication from solid tumors. The incidence of NM has increased as patients are living longer due to significant improvements in treatment options in the form of large molecule target agents. There are case reports about cancers that don’t yet progress into NM, such as ovarian cancer,[1] prostate cancer,[2] and renal cancer.[3] NM is clinically detected in 5-8% of the patients with cancers, while through autopsies NM detected in 19% of the cancerous patients.
Cerebrospinal fluid cytology (CSFC) is the gold standard for determining NM, with the reported sensitivity of CSFC being 71-94%.[4-9] The survival of NM ranges from 8 to 16 weeks despite treatment.[10,11] Patients have a poor Karnofsky Performance Score (KPS), when diagnosed with either bulky central nervous system (CNS) disease, abnormal CSF-flow study, multiple serious neurological deficits, encephalopathy, and extensive systemic cancer without good treatment options have poorer prognosis and need palliative care instead of positive therapy.[9] For an improved outcome, most patients of NM need a combination of radiation therapy, systemic chemotherapy, and intrathecal chemotherapy. Intrathecal chemotherapy is the main treatment of NM. Methotrexate, cytarabine, thiotepa, liposomal cytarabine are the traditional intrathecal chemotherapy regimens.
Intrathecal methotrexate has a long history of treating NM.[12] Intrathecal methotrexate is now widely used to treat NM in the patients with those cancers with possible metastasis to the CNS, such as gastric cancer, breast cancer, lymphoma, nonsmall-cell lung cancer, multiple myeloma, as well as in the patients with cancers rarely spreading to CNS-atypical neurofibroma and with pancreatic cancer.[13-19] Though many physicians use intrathecal cytotoxic drugs in combination with system chemotherapy or target agents,[15,16,20] it is irreplaceable in the treatment of NM, despite of some reported adverse reactions.[21,22] We acquire some clinical experience about how to minimize the side effect and how to institute the course of treatment.
Methods
Inclusion criteria
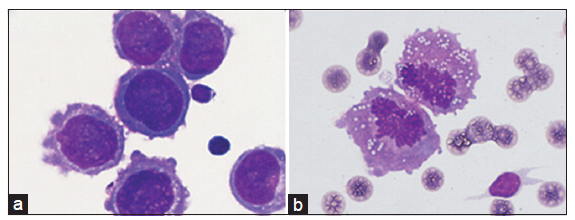
Subjects were required to present with the clinical signs and symptoms consistent with NM, including headache, confusion, cranial and spinal nerve involvement, nausea and vomit. CSF (200 μL) was collected from a lumbar puncture and it was centrifuged in (650 rpm) for 4 min using Slide Centrifuge (Shandon Cytospin 4, Thermo). Cell slides were May-Grunwald-Giemsa stained for 5 min then phosphate buffers was added and incubated for 10 min, followed by gentle rinsing with running water. The stained cell slides were observed under the microscope (Oil immersion lens ×1000, Olympus DP72). NM was diagnosed once tumor cells were found by experienced examiners as showed in Figure 1. Patients with cancer cells were allocated to intrathecal dexamethasone and methotrexate (IT DXM and MTX) group and palliative care group according to their families’ will.
Subjects were discontinuous cases from 2006 to 2014 who did CSF cytologic exams in the CSF cytological examination laboratory of the Second Hospital Affiliated to Hebei Medical University.
Treatment of neoplastic meningitis
After the NM diagnosis, the patients in IT DXM and MTX group received intrathecal dexamethasone 5 mg and methotrexate 10 mg, two doses a week as an inductive treatment of 4 weeks until the symptom was relieved or tumor cells reduced significantly in CSFC examination. Then the patients underwent treatments with a dehydrating agent, pain killer drugs, benzodiazepines, as well as other supportive treatments in the hospital. Then IT DXM and MTX was given one dose every 2 weeks in the outpatient department until the general condition severely deteriorated and could not sustain one’s life. Subjects in the palliative care group received supportive treatments in hospital or at home, according to the families’ determination based on the pain of lumber puncture or economic reasons.
Intrathecal injections were conducted as follows: use of intravenous mannitol 250 mL was 20 min before lumbar puncture and remained throughout the process of lumber puncture. The infusion apparatuses were readily available in case of use of emergency drugs. Ten milliliter CSF was slowly drained out of the subarachnoid space through a half-clogging needle for CSF examination. The needle was then returned into the cannulas. Dexamethasone sodium phosphate was diluted from 1 mL (5 mg) to 5 mL with physiological saline and then slowly injected into the subarachnoid space. During the injection, dexamethasone sodium phosphate was mixed with drawing back CSF repeatedly. Methotrexate was diluted to 5 mL and then injected the same way dexamethasone was treated.
Data collection
The patients’ characteristics and treatment information at the diagnosis of NM were obtained in the medical record from the Second Hospital of Hebei Medical University. Survival data, subsequent therapeutic schedule, and side effects following discharge were obtained by making the phone calls to ask whether there is paralysis, severe vomiting, headache within 48 h after intrathecal injection, or the symptoms of bone marrow suppression, such as fever, infection, and low blood cell count. Overall survival was calculated from the diagnosis of NM.
Results
The patients’ characteristics
Twenty-three subjects were diagnosed as NM according to the positive CSF results as shown in Figure 1. Patient characteristics were summarized in Table 1. Eight patients received IT DXM and MTX treatment as IT DXM and MTX group, and 15 patients as the palliative care group was treated with palliative therapy, such as dehydrant drugs and painkillers.
Figure 1. Neoplastic meningitis was diagnosed when irregular‑shaped cells with big nucleus (a) or high ratio of mitotic cells (b) in cerebrospinal fluid were deep‑stained
The patients' characteristics
| n (ratio or range) | |
|---|---|
| IT DXM and MTX group/palliative care | 8/15 |
| Male/female | 9/14 |
| Age | 55 (21-67) |
| Presenting symptoms | |
| High intracranial pressure | 22 |
| Unable to walk | 3 |
| Visual loss | 9 |
| Hearing damage | 4 |
| Sphincter disturbances | 4 |
| Seizure | 6 |
| Confusion | 3 |
| Cancer type (IT DXM and MTX group/palliative care group) | |
| Lung cancer | 4/6 |
| Breast cancer | 1/2 |
| Gastric cancer | 0/1 |
| Malignant melanoma | 0/1 |
| Unknown | 3/5 |
| Concurrent treatment | |
| IT DXM and MTX | 8 |
| Systemic chemo | 1 |
| WBRT | 2 |
| VP shunt | 1 |
Among them, 22 subjects showed high intracranial pressure (> 200 mmH2O), with other common presenting symptoms including inability to walk (n = 3), varying degrees of visual loss (n = 9), hearing damage (n = 4), sphincter disturbances (n = 4), seizure (n = 6), and confusion (n = 3). Furthermore, 1 patient received systemic chemotherapy, 2 received whole brain radioth=erapy, and 1 received ventriculoperitoneal shunt (VP shunt) treatment. All patients showed positive results by CSFC exam.
Survival
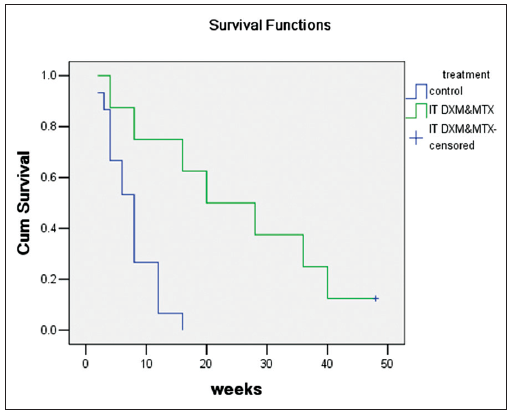
Overall survival (OS) was assessed from the time of NM diagnosis to death and then Kaplan-Meier analysis (group by treatment as IT DXM and MTX group/palliative care group; log-rank test) was conducted [Table 2 and Figure 2]. The OS of IT DXM and MTX group was significantly longer than that of the palliative care group (P = 0.01). The median survival of palliative care group is 7.53 weeks (5.5-9.57; n =15), and have the IT DXM and MTX group, 28.63 weeks (12.50-44.75; n =8), of the total patients, 14.87 weeks (7.93-21.81 weeks; n =23).
Overall survival of different treatments
| Treatment | n | Median OS weeks | 95% CI | P (log-rank) |
|---|---|---|---|---|
| Palliative care group | 15 | 7.53 | 5.50-9.57 | 0.01 |
| IT DXM and MTX | 8 | 28.63 | 12.50-44.75 | |
| Total | 23 | 14.87 | 7.93-21.81 |
Figure 2. Kaplan-Meier analysis of overall survival in intrathecal dexamethasone and methotrexate group and palliative care group
We collected the patients’ characteristics, symptoms, treatment method, and some laboratory examinations at the initial diagnosis of NM, including IT DXM and MTX, KPS, age, gender, primary tumor, cranial nerves damage, spinal nerves damage, seizure, confusion, and level of hemoglobin, albumin, and globulin. We analyzed the possible survival ratio of these factors using Cox’s proportional hazards regression model and the method of forward LR, by entering the factor when P < 0.05 and removing it when P > 0.06. IT DXM and MTX prolonged the OS of NM (regression coefficient = -2.923), odds ratio (OR) = 0.054 (0.09-0.323). Spinal nerves damage decreased the OS (regression coefficient = 1.595), OR = 4.928 (1.382-17.579). Other factors did not enter the Cox’s model (KPS, P = 0.935; age, P = 0.270; gender, P = 0.726; primary tumor, P = 0.220; cranial nerve damage, P = 0.564; seizure, P = 0.605; confusion, P = 0.485; hemoglobin level, P = 0.434; albumin level, P = 0.658; globulin level, P = 0.781).
Bias analysis
There are some innate biases in retrospective studies. Recall bias and confounding bias were the most important biases in our study. Recall bias is innate and uncontrollable, so the conclusion about IT treatment may be not well-grounded. For the latter bias, we analyzed some confounding factors between IT DXM and MTX group and palliative care group. We used KPS as a quantitative index of the subjects’ condition. There were no differences in KPS (2 independent samples t-test, P = 0.733) and gender (Fisher exact t-test, P = 0.367) between the groups, so we can exclude the imbalanced distribution of the KPS and gender and its effects on the different OS between the groups. Age of IT DXM and MTX group is higher than that of palliative group [Mann-Whitney U, P = 0.043; 60.5 (56.5, 64.5) vs. 55 (44, 66)]. Aged patients present negative prognostic factors,[23,24] but IT DXM and MTX group had elder age and longer survival. This is possibly because of the different treatment methods. Given a small number of cases, we just compare the proportion of lung cancer and breast cancer, finding no difference between the groups (Fisher exact t-test, P = 0.685; P = 1.0, respectively), so we conclude that the primary cancer type was at an equilibrium distribution. Moreover, there were some biases coming from the researchers because this study didn’t involve blind method in experimental design.
Discussion
Neoplastic meningitis is a solid tumor at the advanced stage during which patients usually has severe pain and must administrate painkillers frequently. The diagnosis of NM often leads to palliative treatment that is intended to preclude the additional discomfort with aggressive treatment. Meningitis, seizure, vomit or sort of adverse effects were reported in the past studies. In our study, the patients in IT DXM and MTX group show no obvious side effects. This may be caused by a small number of cases, but we think that side effects could be decreased if the drugs were thoroughly diluted and slowly injected according to the course of treatment. Most of the patients’ discomfort was relieved in 3-4 weeks in IT DXM and MTX group with the decreased use of painkillers. IT DXM and MTX is well tolerant despite of the patients’ conditions. However, the patients with abnormal flow studies are associated with poor efficacy and intrathecal chemotherapy toxicity.[25]
Apart from the low drug concentration, we conclude that good tolerance of IT DXM and MTX schedule is related to dexamethasone. Intrathecal steroid therapy can significantly reduce the IL-6 in CSF, a kind of inflammatory factor,[26] so it may reduce nonspecific inflammatory reaction caused by tumor cells or chemotherapy agents. Dexamethasone has been reported as feasible and well tolerated with concomitant intrathecal liposomal cytarabine in patients with acute lymphoblastic leukemia.[27] However, no prospective trials in adults with NM prove beneficial to use of intra-CSF glucocorticoids in combination with intra-CSF chemotherapy.
The natural processes of NM are disastrous if not well treated, for most patients will have a quickly deteriorated condition and die within 2 months. Intrathecal methotrexate is not a new therapy, but the random controlled trial is rare. IT DXM and MTX prolonged the patients’ survival significantly. The medium survival accords with William R and Theodore’s report,[4,11] and is longer than that reported in Glantz’s study,[28] in which most subjects were dominated by breast cancer. This is different from our study in that lung cancer is the dominating subject in our study that represents shorter survival.[29]
Among all those factors, IT DXM and MTX prolong the survival, while spinal nerves damage shortens the OS. Other factors cannot be deemed as having no influence on the OS definitely, although they were not yet fit into the Cox’s proportional hazards regression model. Given the small number of patients, these factors cannot be statistically discarded as a Type II error. Just as in Glantz’s study, an age > 50, performance status ≤ 70%, primary tumor (lung cancer, malignant melanoma), and lack of cytological response present negative prognostic factors.[29]
Methotrexate is a folate anti-metabolite and a S-phase specific cytotoxin with a CSF half-life of 4.5-8.0 h.[24] Therapeutic CSF concentrations obtained in adults and in children of more than 2 years of age are 12 mg IT MTX and 1 μmol/L or more during 48-72 h, respectively.[30] IT DXM and MTX consists of two injections on a weekly basis for 4 weeks as induction treatment, one injection on a weekly basis for 4 weeks as consolidation treatment, one injection on a monthly basis as maintenance treatment until disease progression. The patients’ responses to IT DXM and MTX are different. Some patients’ CSFC remains plenty of tumor cells though induction treatment is accomplished. Other patients’ CSFC shows tumor cells lysis, and only single tumor cells 2 weeks after the initiation of induction treatment. Hence, flexible induction time should be discussed, we recommend two injections on a weekly basis for 3 weeks as induction treatment, and continue the treatment one more week if CSFC does not show a decrease in tumor cells. Should CSF relapse as a symbol of IT DXM and MTX termination? The answer is “no” by our experience. Restarting induction treatment could reduce the tumor cells in CSF with relieved symptoms, but randomized controlled trials with more clinical cases should be conducted to confirm this viewpoint. Moreover, new clinical trials of NM based on a tumor-specific histology are needed to establish the role of IT DXM and MTX treatment.
In conclusion, intrathecal dexamethasone and methotrexate are a safe and effective therapy. Although there are diversified intrathecal agents in recent years, other cytotoxic drugs and targeted agents such as trastuzumab[13] and combined intrathecal chemotherapy[22] prove efficient in treating NM. Thanks to the uncertain properties of new drugs, combined IT DXM and MTX as a basic treatment may be considered to ensure the therapeutic effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1. Miller E, Dy I, Herzog T. Leptomeningeal carcinomatosis from ovarian cancer. Med Oncol 2012;29:2010-5.
2. Orphanos G, Ardavanis A. Leptomeningeal metastases from prostate cancer: an emerging clinical conundrum. Clin Exp Metastasis 2010;27:19-23.
3. Dalhaug A, Haukland E, Nieder C. Leptomeningeal carcinomatosis from renal cell cancer: treatment attempt with radiation and sunitinib (case report). World J Surg Oncol 2010;8:36.
5. Yap HY, Yap BS, Tashima CK, Distefano A, Blumenschein GR. Meningeal carcinomatosis in breast cancer. Cancer 1978;42:283-6.
6. Lee JL, Kang YK, Kim TW, Chang HM, Lee GW, Ryu MH, Kim E, Oh SJ, Lee JH, Kim SB, Kim SW, Suh C, Lee KH, Lee JS, Kim WK, Kim SH. Leptomeningeal carcinomatosis in gastric cancer. J Neurooncol 2004;66:167-74.
7. Nakagawa H, Murasawa A, Kubo S, Nakajima S, Nakajima Y, Izumoto S, Hayakawa T. Diagnosis and treatment of patients with meningeal carcinomatosis. J Neurooncol 1992;13:81-9.
8. Boogerd W, Vroom TM, van Heerde P, Brutel De L a Riviere G, Peterse JL, van der Sande JJ. CSF cytology versus immunocytochemistry in meningeal carcinomatosis. J Neurol Neurosurg Psychiatry 1988;51:142-5.
9. Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol 2010;11:871-9.
10. Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, Fuks J, Portenoy R. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 1990;9:225-9.
11. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 1982;49:759-72.
12. Lampkin BC, Higgins GR, Hammond D. Absence of neurotoxicity following massive intrathecal administration of methotrexate. Case report. Cancer 1967;20:1780-1.
13. Tomita N, Takasaki H, Ishiyama Y, Kishimoto K, Ishibashi D, Koyama S, Ishii Y, Takahashi H, Numata A, Watanabe R, Tachibana T, Ohshima R, Hagihara M, Hashimoto C, Takemura S, Taguchi J, Fujimaki K, Sakai R, Motomura S, Ishigatsubo Y. Intrathecal methotrexate prophylaxis and central nervous system relapse in patients with diffuse large B-cell lymphoma following rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 2015;56:725-9.
14. Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, Heo DS. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74.
15. Jo JC, Kang MJ, Kim JE, Ahn JH, Jung KH, Gong G, Kim HH, Ahn SD, Kim SS, Son BH, Ahn SH, Kim SB. Clinical features and outcome of leptomeningeal metastasis in patients with breast cancer: a single center experience. Cancer Chemother Pharmacol 2013;72:201-7.
16. Marjanovic S, Mijuskovic Z, Stamatovic D, Madjaru L, Ralic T, Trimcev J, Stojanovic J, Radovic V, 2nd. Multiple myeloma invasion of the central nervous system. Vojnosanit Pregl 2012;69:209-13.
17. Tomita H, Yasui H, Boku N, Nakasu Y, Mitsuya K, Onozawa Y, Fukutomi A, Yamazaki K, Machida N, Taku K, Todaka A, Taniguchi H, Tsushima T. Leptomeningeal carcinomatosis associated with gastric cancer. Int J Clin Oncol 2012;17:361-6.
18. Minchom A, Chan S, Melia W, Shah R. An unusual case of pancreatic cancer with leptomeningeal infiltration. J Gastrointest Cancer 2010;41:107-9.
19. Varyani N, Thukral A, Garg S, Gupta KK, Tandon R, Tripathi K. Atypical neurofibroma and osteosclerotic metastasis. Case Rep Oncol Med 2012;2012:301437.
20. Scott BJ, van Vugt VA, Rush T, Brown T, Chen CC, Carter BS, Schwab R, Fanta P, Helsten T, Bazhenova L, Parker B, Pingle S, Saria MG, Brown BD, Piccioni DE, Kesari S. Concurrent intrathecal methotrexate and liposomal cytarabine for leptomeningeal metastasis from solid tumors: a retrospective cohort study. J Neurooncol 2014;119:361-8.
21. Patel A, Ayto R, MacDonald DH. Posterior reversible encephalopathy after intrathecal methotrexate therapy in diffuse large B-cell lymphoma. Br J Haematol 2013;161:607.
23. Herrlinger U, Wiendl H, Renninger M, Forschler H, Dichgans J, Weller M. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer 2004;91:219-24.
25. Mason WP, Yeh SD, DeAngelis LM. 111Indium-diethylenetriamine pentaacetic acid cerebrospinal fluid flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology 1998;50:438-44.
26. Tay AS, Liu EH, Lee TL, Miyazaki S, Nishimura W, Minami T, Chan YH, Low CM, Tachibana S. Cerebrospinal fluid of postherpetic neuralgia patients induced interleukin-6 release in human glial cell-line T98G. Neurochem Int 2013;63:517-21.
27. Valentin A, Troppan K, Pfeilstocker M, Nosslinger T, Linkesch W, Neumeister P. Safety and tolerability of intrathecal liposomal cytarabine as central nervous system prophylaxis in patients with acute lymphoblastic leukemia. Leuk Lymphoma 2014;55:1739-42.
28. Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, Maria B, Lafollette S, Schumann GB, Cole BF, Howell SB. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 1999;5:3394-402.
29. Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol 2010;136:1729-35.
30. Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013;4:S265-88.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Lv WJ, He JY, Zou YL, Liu YJ, Zhang QQ, Liu X, Bu H. Intrathecal dexamethasone and methotrexate treatment of neoplastic meningitis from solid tumors. Neurosciences 2015;2:162-6. http://dx.doi.org/10.4103/2347-8659.160855
AMA Style
Lv WJ, He JY, Zou YL, Liu YJ, Zhang QQ, Liu X, Bu H. Intrathecal dexamethasone and methotrexate treatment of neoplastic meningitis from solid tumors. Neuroimmunology and Neuroinflammation. 2015; 2: 162-6. http://dx.doi.org/10.4103/2347-8659.160855
Chicago/Turabian Style
Lv, Wen-Jing, Jun-Ying He, Yue-Li Zou, Ya-Juan Liu, Qin-Qin Zhang, Xin Liu, Hui Bu. 2015. "Intrathecal dexamethasone and methotrexate treatment of neoplastic meningitis from solid tumors" Neuroimmunology and Neuroinflammation. 2: 162-6. http://dx.doi.org/10.4103/2347-8659.160855
ACS Style
Lv, W.J.; He J.Y.; Zou Y.L.; Liu Y.J.; Zhang Q.Q.; Liu X.; Bu H. Intrathecal dexamethasone and methotrexate treatment of neoplastic meningitis from solid tumors. Neurosciences. 2015, 2, 162-6. http://dx.doi.org/10.4103/2347-8659.160855
About This Article
Copyright
Data & Comments
Data
 Cite This Article 5 clicks
Cite This Article 5 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.