Prognostic significance of neutrophil-to-lymphocyte ratio in glioblastoma
Abstract
Aim: The neutrophil-to-lymphocyte ratio (NLR) has prognostic value in patients with a variety of cancers. The purpose of this study was to investigate the prognostic value of NLR in patients with glioblastoma.
Methods: A prospective study was conducted on patients receiving surgery for glioblastoma. Preoperative NLR was recorded and correlated with other prognostic factors and survival.
Results: Fifty-one patients were included in the study. The mean NLR ratio was 6.7 ± 4.6. Using receiver operating characteristic curve analysis, an NLR cut-off value of 4.7 was determined to best predict survival. Patients with NLR ratios exceeding 4.7 differed significantly from those with NLR ratios ≤ 4.7 and were associated with reduced survival. Patients with gross total tumor excision had a median survival of 18 months, whereas the median survival time was 11 months in patients with subtotal tumor excision. No significant difference in survival was observed with respect to patient age, gender, Karnofsky performance status, or tumor location. Using multivariate analysis, NLR and extent of tumor resection were identified as factors with independent prognostic power.
Conclusion: Neutrophil-to-lymphocyte ratio is an inexpensive, widely available biomarker of glioblastoma aggressiveness and should be used alongside current glioblastoma prognostic factors.
Keywords
Introduction
Glioblastoma is by far the most common type of primary brain tumor that occurs in adults. This devastating disease is usually incurable and, despite aggressive treatment, the median survival time remains in the range of 15 months.[1] Cancer-associated inflammation has been correlated with outcome in patients with cancer.[2-4] Among the various inflammation markers, the neutrophil-to-lymphocyte ratio (NLR) has been examined in a variety of cancers and has been found to be elevated in patients with more advanced or aggressive disease.[5-8] The exact mechanisms by which neutrophilia is induced by tumors is unclear.[9,10] The secretion of angiogenesis factors and cytokines has been implicated to play a role in neutrophilia induction.[11,12] In gliomas, lymphocyte infltration around the tumor has been associated with a better prognosis.[13] To the best of our knowledge, only one study has assessed the role of NLR in glioblastoma patients.[14] In this study, we aimed to assess the prognostic value of NLR and correlate it with other prognostic factors of glioblastoma.
Methods
Study population
We prospectively studied patients who received surgery for glioblastoma in our institute between March 2007 and September 2013. Patients were included if they had full blood count results at first presentation, before any treatment. The extent of resection was determined by comparing magnetic resonance imaging (MRI) scans obtained before surgery with those obtained within the 1st month after surgery. Clinical variables that were analyzed included age, sex, and preoperative Karnofsky performance status score (KPS). Radiological variables included tumor lateralization, location and volume. Tumor volumes were approximated from preoperative, postgadolinium T1-weighted MRI using a modified ellipsoid volume equation (radiusx × radiusy × radiusz)/2.[15] All patients received postoperative radiotherapy with temozolomide, followed by temozolomide chemotherapy for up to 1-year or until recurrence. Radiotherapy was administered as fractionated focal irradiation at a dose of 2 Gy/fraction given once a day for 5 days/week over a period of 6 weeks up to a total dose of 60 Gy. Follow-up MRIs were performed every 2 months. Recurrence was defined based on MRI and/or single photon emission tomography findings.[16] The study was approved by the Institutional Review Board.
Statistical analysis
Pearson’s correlation coefficient was used to assess continuous variables. Progression-free survival (PFS) was defined as the time from the initial surgery to demonstration of tumor progression on follow-up MRI or to death. Survival time was defined as the time between the date of diagnosis and the date of death for deceased patients, or to the last follow-up for surviving patients. The overall survival time was estimated using Kaplan-Meier methods, and log-rank analysis was performed to compare survival curves between groups. Patients who were still alive at last contact were treated as censored events in the analysis. Multivariate Cox regression analysis of the data was used to analyze possible prognostic factors. The forward step-wise model selection procedure was used (P value of likelihood-ratio test < 0.05 as inclusion criteria; likelihood-ratio test > 0.10 as exclusion criteria) to define the final model. The following variables were entered: gender, age at diagnosis, KPS, NLR, and the extent of resection. With respect to NLR, receiving operating characteristics (ROC) curve analysis was performed in order to determine the cut-off value for predicting survival. A 2-sided P < 0.05 was considered as statistically significant.
Results
Study population
Table 1 summarizes the patient data. Fifty-one patients (30 males, 21 females, mean age 59.2 ± 14.2) met the inclusion criteria for the study. The majority of glioblastomas were lateralized, with 28 (54.9%) on the left and 21 (41.1%) on the right side. The most common tumor site was the temporal lobe (37.2%), followed by the occipital lobe (27.5%). The mean tumor volume was 32.1 ± 27.3 cm3. In 19 cases (37.2%), the tumor was located close to a ventricle. Thirty-four patients had a KPS over 80. In 32 cases, gross total excision was achieved, whereas in 19 cases there was subtotal tumor resection. One patient was lost to follow-up, and one patient died in the immediate postoperative period. After a mean follow-up period of 17 months (range: 3-39 months), 14 patients were alive.
Patient data
| Patient characteristic | n (%) | OS |
|---|---|---|
| P | ||
| Gender | 0.3 | |
| Male | 30 (58.8) | |
| Female | 21 (41.2) | |
| Age | 0.4 | |
| > 60 | 20 (39.2) | |
| < 60 | 31 (60.8) | |
| KPS | 0.052 | |
| > 80 | 34 (66.7) | |
| < 80 | 17 (33.3) | |
| NLR | 0.01 | |
| > 4.7 | 29 (56.8) | |
| < 4.7 | 22 (43.2) | |
| Extent of resection | 0.036 | |
| GTR | 32 (62.7) | |
| STR | 19 (37.2) | |
Neutrophil-to-lymphocyte ratio
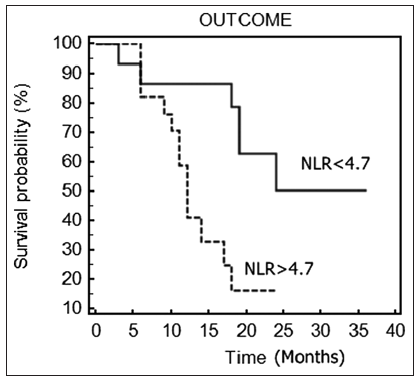
The mean NLR was 6.7 ± 4.6. Using ROC curve analysis, a cut-off NLR value of 4.7 was determined to best predict survival. Patients with an NLR exceeding 4.7 differed significantly from those with an NLR ≤ 4.7 and were associated with decreased survival time (11 vs. 18.7 months, P = 0.01) [Figure 1]. There was a significant increase in PFS for patients with an NLR lower than 4.73 (P = 0.03).
Extent of resection
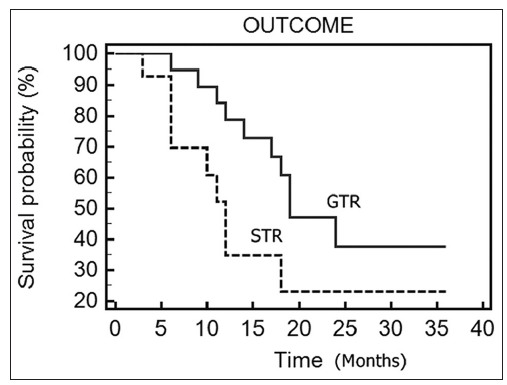
Patients with gross total tumor excision had a median survival of 18 months, whereas in patients with subtotal tumor excision, the median survival time was 11 months. The difference was statistically significant (P = 0.036) [Figure 2].
Karnofsky performance status score
The median survival for patients with KPS over 80 was 17 months, whereas survival for patients with KPS under or equal to 80 was 11 months. However, the difference was marginally significant (P = 0.052). No significant difference in survival was observed with respect to patient age (P = 0.4) or gender (P = 0.3).
Tumor characteristics
In 19 cases, the tumor was located close to a ventricle.These patients were associated with reduced survival (P = 0.052). No prognostic significance was found for tumor location or laterality [Table 2]. No correlation was found between NLR and tumor volume.
Tumor characteristics
| Parameter | Proportion |
|---|---|
| Tumor location (%) | |
| Frontal | 15.7 |
| Temporal | 37.2 |
| Parietal | 13.7 |
| Occipital | 27.5 |
| Multifocal | 5.8 |
| Laterality (%) | |
| Right | 41.1 |
| Left | 54.9 |
| Midline | 4 |
| Located near ventricle (%) | 37.2 |
| Tumor volume (cm3) | 32.1 ± 27.3 |
Multivariate analysis
Using multivariate analysis, NLR (P = 0.011, 95% confidence intervals [CI]: 1.4-17.3) and extent of tumor resection (P = 0.025, 95% CI: 1.2-8.7) were identified as factors with independent prognostic power.
Discussion
In the present study, we found that patients with an NLR over 4.7 were associated with reduced median overall survival. Patients with subtotal tumor excision had reduced median overall survival compared with patients with gross total tumor excision. There was a trend toward increased survival for patients with KPS over 80 and tumors not related to the ventricular system.
In addition to genetic factors, systemic inflammatory response has also been implicated in carcinogenesis. Bambury et al.[1] also studied the prognostic impact of the NLR in a cohort of patients with glioblastoma. The authors studied 84 patients that had full blood count results available at first presentation with symptoms of glioblastoma, and the NLR was calculated. The results of this study showed that age over 65 years, gender, eastern cooperative oncology group performance status ≥ 2, frontal tumor, extent of surgical resection, completion of the adjuvant chemoradiation protocol, and NLR > 4 were significantly correlated with overall survival. The present study verified the above findings. Furthermore, we found no correlation between NLR and tumor volume. Patients with tumors not related to the ventricular system had a better prognosis.
In other cancer studies, a prognostic significance of NLR was found. A recent meta-analysis of 26 studies in primary liver cancer demonstrated that the high NLR can strongly predict poor survival in these patients, indicating the predictive value of the NLR as a new biomarker in primary liver cancer.[6] Furthermore, high NLR was associated with vascular invasion and correlated with alpha-fetoprotein levels. Proctor et al.[12] studied 12,118 patients who had been sampled within 2 years of their cancer diagnosis and found that NLR was independently associated with survival in all cancers studied.
Apart from NLR, other inflammatory markers have also been associated with patient prognosis. Steffens et al.[17] have reported that a high preoperative serum C-reactive protein level is an independent predictor of poor survival in patients with renal cell carcinoma. In the present study, we verified the prognostic significance of gross total tumor excision. This is a well-established prognostic factor.[18] Karnofsky performance scale and age have been also associated with glioblastoma prognosis.[14,18] In the present study, there was a trend towards increased survival for patients with KPS over 80. Glioblastomas adjacent to the lateral ventricles have been suggested to harbor a dismal prognosis.[19,20] Neural and cancer stem cells have been found in the subventricular zone that lines the lateral ventricles.[20] Thus, tumors in this region may be more invasive with higher potential to recruit migratory progenitor cells.[20] In the present study, we verified the prognostic significance of tumors located adjacent to the ventricles.
In conclusion, our results are in agreement with Bambury et al.,[2,3] as well as other reports of the prognostic significance of NLR in a variety of cancers. NLR is an inexpensive and widely available biomarker of glioblastoma aggressiveness, and thus should be used alongside current glioblastoma prognostic factors. Nevertheless, there is obviously a need for future studies with larger numbers of patients in order to confirm our preliminary observations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1. Liu Y, Shete S, Etzel CJ, Scheurer M, Alexiou G, Armstrong G, Tsavachidis S, Liang FW, Gilbert M, Aldape K, Armstrong T, Houlston R, Hosking F, Robertson L, Xiao Y, Wiencke J, Wrensch M, Andersson U, Melin BS, Bondy M. Polymorphisms of LIG4, BTBD2, HMGA2, and RTEL1 genes involved in the double-strand break repair pathway predict glioblastoma survival. J Clin Oncol 2010;28:2467-74.
2. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30.
3. Yuan D, Zhu K, Li K, Yan R, Jia Y, Dang C. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol 2014;110:333-40.
4. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Oca-a A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124.
5. Lang BH, Ng CP, Au KB, Wong KP, Wong KK, Wan KY. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg 2014;38:2605-12.
6. Xue TC, Zhang L, Xie XY, Ge NL, Li LX, Zhang BH, Ye SL, Ren ZG. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: a meta-analysis. PLoS One 2014;9:e96072.
7. Ozdemir Y, Akin ML, Sucullu I, Balta AZ, Yucel E. Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pac J Cancer Prev 2014;15:2647-50.
8. Chang Z, Zheng J, Ma Y, Zhao J, Wang C, Liu Z. The neutrophil-to-lymphocyte ratio as a predictor for recurrence of colorectal liver metastases following radiofrequency ablation. Med Oncol 2014;31:855.
9. Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014;14:117.
10. Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, Cramer DW. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol 2014;132:542-50.
11. Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther 2013;6:1605-12.
12. Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 2012;107:695-9.
13. Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer 2014;110:2560-8.
14. Bambury RM, Teo MY, Power DG, Yusuf A, Murray S, Battley JE, Drake C, O'Dea P, Bermingham N, Keohane C, Grossman SA, Moylan EJ, O'Reilly S. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol 2013;114:149-54.
15. Alexiou GA, Vartholomatos G, Karamoutsios A, Batistatou A, Kyritsis AP, Voulgaris S. Circulating progenitor cells: a comparison of patients with glioblastoma or meningioma. Acta Neurol Belg 2013;113:7-11.
16. Alexiou GA, Zikou A, Tsiouris S, Goussia A, Kosta P, Papadopoulos A, Voulgaris S, Tsekeris P, Kyritsis AP, Fotopoulos AD, Argyropoulou MI. Comparison of diffusion tensor, dynamic susceptibility contrast MRI and (99m) Tc-Tetrofosmin brain SPECT for the detection of recurrent high-grade glioma. Magn Reson Imaging 2014;32:854-9.
17. Steffens S, Köhler A, Rudolph R, Eggers H, Seidel C, Janssen M, Wegener G, Schrader M, Kuczyk MA, Schrader AJ. Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC Cancer 2012;12:399.
18. Alexi ou GA, Gou ssia A, Vou lga ris S, Fotop ou los AD, Fotakopoulos G, Ntoulia A, Zikou A, Tsekeris P, Argyropoulou MI, Kyritsis AP. Prognostic significance of MRP5 immunohistochemical expression in glioblastoma. Cancer Chemother Pharmacol 2012;69:1387-91.
19. Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol 2008;89:219-24.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Alexiou GA, Vartholomatos E, Zagorianakou P, Voulgaris S. Prognostic significance of neutrophil-to-lymphocyte ratio in glioblastoma. Neurosciences 2014;1:131-4. http://dx.doi.org/10.4103/2347-8659.143666
AMA Style
Alexiou GA, Vartholomatos E, Zagorianakou P, Voulgaris S. Prognostic significance of neutrophil-to-lymphocyte ratio in glioblastoma. Neuroimmunology and Neuroinflammation. 2014; 1: 131-4. http://dx.doi.org/10.4103/2347-8659.143666
Chicago/Turabian Style
Alexiou, George A., Evrysthenis Vartholomatos, Panagiota Zagorianakou, Spyridon Voulgaris. 2014. "Prognostic significance of neutrophil-to-lymphocyte ratio in glioblastoma" Neuroimmunology and Neuroinflammation. 1: 131-4. http://dx.doi.org/10.4103/2347-8659.143666
ACS Style
Alexiou, GA.; Vartholomatos E.; Zagorianakou P.; Voulgaris S. Prognostic significance of neutrophil-to-lymphocyte ratio in glioblastoma. Neurosciences. 2014, 1, 131-4. http://dx.doi.org/10.4103/2347-8659.143666
About This Article
Copyright
Data & Comments
Data
 Cite This Article 1 clicks
Cite This Article 1 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.